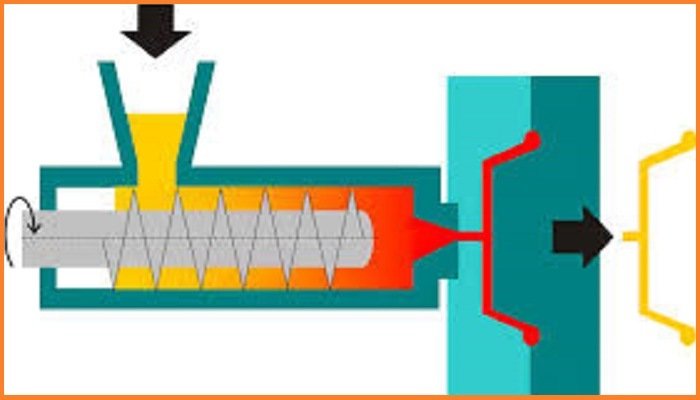
PVC TECHNOLOGY
VINYL CHLORIDE MONOMER (VCM)

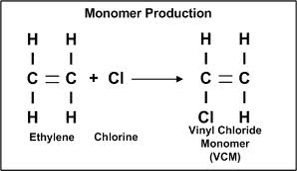
MONOMER PRODUCTION
VCM PRODUCTION

VCM PRODUCTION PROCESS
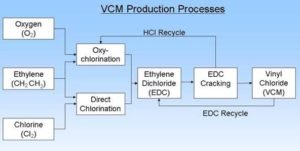
VCM is produced by Dow by cracking ethylene dichloride (EDC). EDC is produced by the chlorination of ethylene through one of two processes: direct chlorination or oxychlorination. The direct chlorination process reacts ethylene with chlorine, while oxychlorination reacts ethylene with dry hydrogen chloride and oxygen, to produce EDC, as shown in the process diagram.
VINYL CHLORIDE
- Molecular Weight 62.5
- Boiling point -13.9 degree C
- Flash Point (starting of vapour) -77.8 degree C
- Freezing Point -153.8 degree C
VCM, which is the intermediate raw material for PVC, has a boiling point of – 13.9°C and a flash point of – 78°C. Caution is required upon handling since it is a dangerous substance in gaseous form. The PVC industry in Europe handles VCM with utmost care at PVC manufacturing facilities and ensures a safe working environment.
Although VCM is a dangerous substance in terms of flammability and reactivity it can be distributed and handled safely provided that appropriate precautions are observed.
Distribution is already subject to regulations within most countries in Europe. In additional, international movement by road, rail, sea and inland waterway is subject to agreements that lay down specific requirements that must be observed by all parties involved. National regulations may contain additional requirements.
As part of its commitment to the Responsible Care© programme of the European Chemical Industry Council (Cefic), the PVC industry has prepared additional guidelines. These cover all aspects of transport activity from loading to delivery point.
A copy of the full guidelines can be obtained from the ECVM secretariat. The PVC industry is also endeavoring to reduce as much as possible the (already low) exposure of local populations to VCM.
PVC POLYMERIZATION
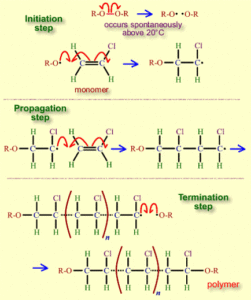
SUSPENSION POLYMERIZATION TECHNIQUE

TEMP. 50-70 DEGREE C , PRESSURE 250 PSI, 8VC MONOMER join to gather in growing pvc molecules, it precipitates.
HIGH TEMP. –HIGH K VALUE, HIGH CRYSTALLINE, HIGH Tg.
By far the most widely used production process is suspension polymerization. In this process, VCM and water are introduced into the polymerization reactor and a polymerization initiator, along with other chemical additives, are added to initiate the polymerization reaction. The contents of the reaction vessel are continually mixed to maintain the suspension and ensure a uniform particle size of the PVC resin. The reaction is exothermic, and thus requires a cooling mechanism to maintain the reactor contents at the appropriate temperature. As the volumes also contract during the reaction (PVC is denser than VCM), water is continually added to the mixture to maintain the suspension.
Once the reaction has run its course, the resulting PVC slurry is degassed and stripped to remove excess VCM (which is recycled) then passed though a centrifuge to remove water. The slurry is further dried in a hot air bed, and the resulting powder sieved before storage or palletization. Normally, the resulting PVC has a VCM content of less than 1 part per million.
Other production processes, such as micro-suspension polymerization and emulsion polymerization, produce PVC with smaller particle sizes (10 μm vs. 120-150 μm for suspension PVC) with slightly different properties and with somewhat different sets of applications.
The product of the polymerization process is unmodified PVC. Before PVC can be made into finished products, it almost always requires conversion into a compound by the incorporation of additives such as heat stabilizers, UV stabilizers, lubricants, plasticizers, processing aids, impact modifiers, thermal modifiers, fillers, flame retardants, biocides, blowing agents and smoke suppressors, and, optionally pigments.
TEMP. 50-70 DEGREE C , PRESSURE 250 PSI, 8VC MONOMER join to gather in growing pvc molecules, it precipitates.
HIGH TEMP. –HIGH K VALUE, HIGH CRYSTALLINE, HIGH Tg.
PVC PROPERTIES
- Polyvinyl chloride
- Elongation at break
- 20-40%
- Notch test
- 2-5 kj/m2
- Glass Temperature
- 82 Degree C
- Melting Point
- 100–260 °C
- Water absorption (ASTM)
- 0.04-0.4
PROPERTIES RIGID
- Density [g/cm3]
- 1.3–1.45 1.1–1.35
- Thermal Conductivity [W/(m·K)]
- 0.14–0.28 0.14–0.17
- Yield Strength [MPa]
- 31–60 10–25
- Young’s Modulus [psi]
- 490,000
- Flexural Strength (yield) [psi]
- 10,500
- Compression strength [psi]
- 9500
PROPERTIES OF FLEXIBLE PVC
- Coefficient of thermal expansion (linear) [mm/(mm °C)]
- 5×10−5
- Vicat B [°C]
- 65–100
- Flexural strength- Not recommended
- Resistivity [Ω m]
- 1016 1012–1015
- Surface Resistivity [Ω]
- 1013–1014 1011–1012
Mass Polymerization of Polyvinylchloride
- The mass polymerization involves no solvents or second dispersing phase. Typically mass polymerization of
Polyvinylchloride is done in a two different vessels called pre-polymerizer and post-polymerizer. The polymerization is carried out up to about 10 – 12% in the pre-polymerizer, where the agitation( uneven) speed and temperature is controlled to adjust the grain size and porosity of the final resin. Then the free flowing mass called seed particles are transformed to the post-polymerizer which holds additional monomer and initiator. Mass polymerization at post-polymerizer is usually done at 50-60 °C, and driven by high pressure rather than temperature. Controlling the final molecular weight is done at the post-polymerizer. The advantages of using mass polymerization technique includes, no involment with water, no pericelluar membrane in resin particles which in turn makes it easier for absorbing other additives including plasticizers, energy conservation as the drying step doesn’t involve applying large amounts of heat etc. The major drawbacks are the presence of large amounts of fine particles which creates dusting issues and process issues for some industries, need to apply high heat at stripping to get down the vinyl chloride monomer content to the required 1 ppm level etc.
Emulsion and Microsuspension Polymerization of Polyvinylchloride
Emulsion polymerization of vinyl chloride had being the most predominant method for producing Polyvinylchloride until the suspension process was developed. In the emulsion polymerization, emulsifies (soap) are used to disperse the vinyl chloride monomer in water, where as suspending agents are used in suspension polymerization. Two main routes of emulsion polymerization of vinyl chloride includes microsuspension and conventional emulsion polymerization. Both methods produce nonporous very fine grains in the range of 1 micron, where as the mass and suspension processes produce much larger particles. The ability to make fine grains has the advantage of making films and products without the graininess-surface.
Conventional emulsion polymerization of vinyl chloride involves using a surfactants, usually anionic surfactants such as sodium lauryl sulfate, to increase the surface tension and conductivity until it reaches the critical micelle concentration (c.m.c). At this stage, the emulsifies molecules are arranged in way that their hydrophilic groups are at the outer space contacting with water, and hydrophobic groups are gathered inside the micelle. Most of the vinyl chloride monomer droplets get dispersed in the micelle. For the conventional emulsion polymerization, water soluble free radical initiators are used, as opposed to monomer-soluble initiators used in suspension polymerization. When a free radical generated in water enters the micelle containing monomer droplets, polymerization get initiated. Usually the particle size of the resulting resin would be less than 0.5 microns. In order to get larger particles a seeding technique is usually used, where the polymerized batch gets transferred to another vessel where more monomer is added and the polymerization continues During the microsuspension polymerization, monomer soluble initiators are used as in the suspension technique.
Although the microsuspension process uses a high load of the emulsifier, it doesn’t require the formation of
micelles, and rely on the use of a homogenizer — colloid mill, high speed pump or an ultrasonic device–, to make
submicron size monomer droplets.
The particle properties of the PVC made by emulsion and microsuspension methods depends on process variables including emulsifier system, the type of the electrolytes used, homogenization process, seeding technique as well as the spray drying process used. Although it is very costly to make the resins by these techniques, the ability to make fine particle makes it useful to be used them in plastisol applications. Usually a combinations of PVC resins with various particle size ranges are blended together to produce the grade suitable for plastisol applications.
Molecular Weight Distribution
The distribution of different sized molecules in a pvc polymer typically follows the bell shaped normal distribution curve described by the Gaussian probability theory. As with other populations, the bell shaped curve can reflect distributions ranging from narrow to broad. A polymer containing a broad range of chain lengths is said to have a broad molecular weight distribution (MWD). Resins with this type of distribution have good environmental Stress Crack Resistance (ESCR), good impact resistance and good processability. A polymer with a narrow MWD contains molecules that are nearly the same in molecular weight. It will crystallize at a faster, more uniform rate. This results in a product that will hold its shape. Polymers can also have a bimodal shaped distribution curve which, as the name suggests, seem to depict a blend of two different polymer populations, each with its particular average and distribution. Resins having a bimodal MWD contain both very short and very long pvc molecules, giving the resin excellent physical properties while maintaining good process-ability.
MWD is dependent upon the type of process used to manufacture the particular PVC resin. For polymers of the same density and average molecular weight, their melt flow rates are relatively independent of MWD. Therefore, resins that have the same density and melt index (MI) can have very different molecular weight distributions. The effects of density, molecular weight and molecular weight.