Polyvinyl Chloride (PVC)
Chemical Formula: (C2H3Cl)n
Poly vinyl chloride is also known as poly vinyl or Vinyl, As abbreviated known as PVC. PVC is the widely third most, large using synthetic polymer in world followed by Polyethylene & Polypropene.
Polyvinyl chloride is a white, brittle solid. It is insoluble in alcohol and slightly soluble in tetrehydrofuran.
Production
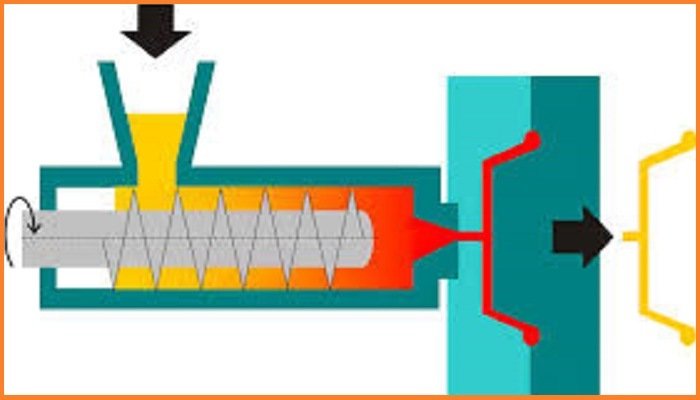
Polyvinyl chloride is produced by polymerization of the vinyl chloride monomer (VCM).
About 80% of production involves suspension polymerization. Emulsion polymerization accounts for about 12%, and bulk polymerization accounts for 8%. Suspension polymerization affords particles with average diameters of 100–180 μm, whereas emulsion polymerization gives much smaller particles of average size around 0.2 μm. VCM and water are introduced into the reactor along with a polymerization initiator and other additives. The contents of the reaction vessel are pressurized and continually mixed to maintain the suspension and ensure a uniform particle size of the PVC resin. The reaction is exothermic and thus requires cooling. As the volume is reduced during the reaction (PVC is denser than VCM), water is continually added to the mixture to maintain the suspension.
The polymerization of VCM is started by compounds called initiators that are mixed into the droplets. These compounds break down to start the radical chain reaction. Typical initiators include dioctanoyl peroxide and dicetyl peroxydicarbonate, both of which have fragile O-O bonds. Some initiators start the reaction rapidly but decay quickly, and other initiators have the opposite effect. A combination of two different initiators is often used to give a uniform rate of polymerization. After the polymer has grown by about 10 times, the short polymer precipitates inside the droplet of VCM, and polymerization continues with the precipitated, solvent-swollen particles. The weight average molecular weights of commercial polymers range from 100,000 to 200,000, and the number average molecular weights range from 45,000 to 64,000.
Once the reaction has run its course, the resulting PVC slurry is degassed and stripped to remove excess VCM, which is recycled. The polymer is then passed through a centrifuge to remove water. The slurry is further dried in a hot air bed, and the resulting powder is sieved before storage or pelletization. Normally, the resulting PVC has a VCM content of less than 1 part per million. Other production processes, such as micro-suspension polymerization and emulsion polymerization, produce PVC with smaller particle sizes (10 μm vs. 120–150 μm for suspension PVC) with slightly different properties and with somewhat different sets of applications.
Additives
The product of the polymerization process is unmodified PVC. Before PVC can be made into finished products, it always requires conversion into a compound by the incorporation of additives (but not necessarily all of the following) such as heat stabilizers, UV stabilizers, plasticizers, processing aids, impact modifiers, thermal modifiers, fillers, flame retardants, biocides, blowing agents and smoke suppressors, and, optionally, pigments. The choice of additives used for the PVC finished product is controlled by the cost performance requirements of the end use specification (underground pipe, window frames, intravenous tubing and flooring all have very different ingredients to suit their performance requirements). Previously, polychlorinated biphenyls (PCBs) were added to certain PVC products as flame retardants and stabilizer.
Phthalate plasticizers
Most vinyl products contain plasticizers which dramatically improve their performance characteristic. The most common plasticizers are derivatives of phthalic acid. The materials are selected on their compatibility with the polymer, low volatility levels, and cost. These materials are usually oily colourless substances that mix well with the PVC particles. About 90% of the plasticizer market, estimated to be millions of tons per year worldwide, is dedicated to PVC.
Metal stabilizers
Liquid mixed metal stabilisers are used in several PVC flexible applications such as calendered films, extruded profiles, injection moulded soles and footwear, extruded hoses and plastisols where PVC paste is spread on to a backing (flooring, wall covering, artificial leather). Liquid mixed metal stabiliser systems are primarily based on barium, zinc and calcium carboxylates. In general liquid mixed metals like BaZn, CaZn require the addition of co-stabilisers, antioxidants and organo-phosphites to provide optimum performance.
BaZn stabilisers have successfully replaced cadmium-based stabilisers in Europe in many PVC semi-rigid and flexible applications.
Heat stabilizers
One of the most crucial additives are heat stabilizers. These agents minimize loss of HCl, a degradation process that starts above 70 °C. Once dehydrochlorination starts, it is autocatalytic. Many diverse agents have been used including, traditionally, derivatives of heavy metals (lead, cadmium). Increasingly, metallic soaps (metal “salts” of fatty acids) are favored, species such as calcium stearate. Addition levels vary typically from 2% to 4%. The choice of the best heat stabilizer depends on its cost effectiveness in the end use application, performance specification requirements, processing technology and regulatory approvals.
Di-2ethylhexylphthalate
Di-2ethylhexylphthalate (DEHP) has been medically approved for many years for use in medical devices; the PVC-DEHP combination proving to be very suitable for making blood bags because DEHP stabilizers red blood cells, minimizing haemolysis (red blood cell rupture). However, DEHP is coming under increasing pressure in Europe. The assessment of potential risks related to phthalates, and in particular the use of DEHP in PVC medical devices, was subject to scientific and policy review by the European Union authorities, and on 21 March 2010, a specific labeling requirement was introduced across the EU for all devices containing phthalates that are classified as CMR (carcinogenic, mutagenic or toxic to reproduction). The label aims to enable healthcare professionals to use this equipment safely, and, where needed, take appropriate precautionary measures for patients at risk of over-exposure.
DEHP alternatives, which are gradually replacing it, are Adipates, Butyryltrihexylcitrate (BTHC), Cyclohexane-1,2-dicarboxylic acid, diisononylester (DINCH), Di(2-ethylhexyl)terephthalate, polymerics and trimellitic acid, 2-ethylhexylester (TOTM).
Properties
PVC is a thermoplastic polymer. Its properties are usually categorized based on rigid and flexible PVCs.
| Properties | Rigid PVC | Flexible PVC |
| Density (g/cc) | 1.3 – 1.45 | 1.1 – 1.35 |
| Thermal conductivity (w/mK) | 0.14 – 0.28 | 0.14 – 0.17 |
| Yield Strength (psi) | 4500 – 8700 | 1650 – 3600 |
| Young’s Modulus (psi) | 490000 | – |
| Flexural Strength (psi) | 10500 | – |
| Compression Strength (psi) | 9500 | – |
| Co-efficient of liner thermal expansion (mm/mm deg C) | 5 x 10 -5 | – |
| Vicat softening Temperature | 65 – 100 deg C | – |
| Heat Distortion temp. Deg C | 70 – 100 | – |
| Resistivity (Ohm.m) | 10 16 | 10 12 – 10 15 |
| Surface Resistivity (Ohm) | 1013 – 1014 | 1011 – 1012 |
| Elongation at break | 30 – 40% | 40 – 70% |
| Notch test (Kj/m2) | 2 – 5 | – |
| Melting Point (Deg C) | 130 – 260 | 100 – 260 |
| Water Absorption | 0.04 – 4 |
Mechanical
PVC has high hardness and mechanical properties. The mechanical properties enhance with the molecular weight increasing but decrease with the temperature increasing. The mechanical properties of rigid PVC (uPVC) are very good; the elastic modulus can reach 1500-3,000 MPa. The soft PVC (flexible PVC) elastic is 1.5–15 MPa.
Thermal and fire
The heat stability of raw PVC is very poor, so the addition of a heat stabilizer during the process is necessary in order to ensure the product’s properties. PVC starts to decompose when the temperature reaches 140 °C (284 °F), with melting temperature starting around 160 °C (320 °F). The linear expansion coefficient of rigid PVC is small and has good flame retardancy, the Limiting oxygen index (LOI) being up to 45 or more. The LOI is the minimum concentration of oxygen, expressed as a percentage, that will support combustion of a polymer and noting that air has 20% content of oxygen.
Electrical
PVC is a polymer with good insulation properties, but because of its higher polar nature the electrical insulating property is inferior to non polar polymers such as polyethylene and polypropylene. Since the dielectric constant, dielectric loss tangent value, and volume resistivity are high, the corona resistance is not very good, and it is generally suitable for medium or low voltage and low frequency insulation materials.
Chemical
PVC is chemically resistant to acids, salts, bases, fats, and alcohols; therefore, it is used in sewerage piping. It is also resistant to some solvents, mainly uPVC. Plasticized PVC, also known as PVC-P, is in some cases less resistant to solvents. For example, PVC is resistant to fuel and some paint thinners. Some solvents may only swell it or deform it but not dissolve it, but some of them, like tetrahydrofuran or acetone, may damage it.
Application
Pipe, Flooring, Furniture, Structure, wire ropes, healthcare & construction etc.
Electric cables
PVC is commonly used as the insulation on electrical cables; PVC used for this purpose needs to be plasticized.
Flexible PVC coated wire and cable for electrical use has traditionally been stabilised with lead, but these are being replaced with calcium-based systems. In a fire, PVC-coated wires can form hydrogen chloride fumes; the chlorine serves to scavenge free radicals and is the source of the material’s fire retardance. While hydrogen chloride fumes can also pose a health hazard in their own right, it dissolves in moisture and breaks down onto surfaces, particularly in areas where the air is cool enough to breathe, and is not available for inhalation. Frequently in applications where smoke is a major hazard (notably in tunnels and communal areas), PVC-free cable insulation is preferred, such as low smoke zero halogen (LSZH) insulation.
Construction
PVC is a common, strong but lightweight plastic used in construction. It is made softer and more flexible by the addition of plasticizers. If no plasticizers are added, it is known as uPVC (unplasticized polyvinyl chloride) or rigid PVC.
uPVC is extensively used in the building industry as a low-maintenance material, particularly in Ireland, the United Kingdom, in the United States and Canada. In the U.S. and Canada it is known as vinyl or vinyl siding. The material comes in a range of colors and finishes, including a photo-effect wood finish, and is used as a substitute for painted wood, mostly for window frames and sills when installing insulated glazing in new buildings, or to replace older single-glazed windows. Other uses include fascia, and siding or weatherboarding. This material has almost entirely replaced the use of cast iron for plumbing and drainage, being used for waste pipes, drainpipes, gutters and downspouts. uPVC is known as having strong resistance against chemicals, sunlight, and oxidation from water.
Signs
Polyvinyl chloride is formed in flat sheets in a variety of thicknesses and colors. As flat sheets, PVC is often expanded to create voids in the interior of the material, providing additional thickness without additional weight and minimal extra cost (see Closed-cell PVC foamboard). Sheets are cut using saws and rotary cutting equipment. Plasticized PVC is also used to produce thin, colored, or clear, adhesive-backed films referred to simply as vinyl. These films are typically cut on a computer-controlled plotter (see Vinyl cutter) or printed in a wide-format printer. These sheets and films are used to produce a wide variety of commercial signage products, including car body stripes and stickers.
Clothing and furniture
PVC has become widely used in clothing, to either create a leather-like material or at times simply for the effect of PVC. PVC clothing is common in Goth, Punk, clothing fetish and alternative fashions. PVC is less expensive than rubber, leather, and latex which it is used to simulate.
PVC fabric is water-resistant, so it is used in coats, skiing equipment, shoes, jackets, aprons, and bags
Healthcare
The two main application areas for single-use medically approved PVC compounds are flexible containers and tubing: containers used for blood and blood components, for urine collection or for ostomy products and tubing used for blood taking and blood giving sets, catheters, heart-lung bypass sets, hemodialysis sets etc. In Europe the consumption of PVC for medical devices is approximately 85,000 tons each year. Almost one third of plastic-based medical devices are made from PVC. The reasons for using flexible PVC in these applications for over 50 years are numerous and based on cost effectiveness linked to transparency, light weight, softness, tear strength, kink resistance, suitability for sterilization and biocompatibility.
Flooring
Flexible PVC flooring is inexpensive and used in a variety of buildings covering the home, hospitals, offices, schools, etc. Complex and 3D designs are possible, which are then protected by a clear wear layer. A middle vinyl foam layer also gives a comfortable and safe feel. The smooth, tough surface of the upper wear layer prevents the buildup of dirt, which prevents microbes from breeding in areas that need to be kept sterile, such as hospitals and clinics.
Wire rope
PVC coating placed onto wire rope and aircraft cable is used for general purpose applications. The coating process consists of a jacketing application via pressurized extrusion. The benefits of PVC coating on wire rope are for not only aesthetics, but for ergonomics, abrasion protection and visibility. It is found in a variety of industries and environments both in-door and out.
Other applications
PVC has been used for a host of consumer products. One of its earliest mass-market consumer applications was vinyl record production. More recent examples include wallcovering, greenhouses, home playgrounds, foam and other toys, custom truck toppers (tarpaulins), ceiling tiles and other kinds of interior cladding.
PVC piping is cheaper than metals used in musical instrument making; it is therefore a common alternative when making instruments, often for leisure or for rarer instruments such as the contrabass flute.
Chlorinated PVC
PVC can be usefully modified by chlorination, which increases its chlorine content to 67%. The new material has a higher heat resistance so is primarily used for hot water pipe and fittings, but it is more expensive and it is found only in niche applications, such as certain water heaters and certain specialized clothing. An extensive market for chlorinated PVC is in pipe for use in office building, apartment and condominium fire protection. CPVC, as it is called, is produced by chlorination of aqueous solution of suspension PVC particles followed by exposure to UV light which initiates the free-radical chlorination.
Degradation
Degradation during service life, or after careless disposal, is a chemical change that drastically reduces the average molecular weight of the polyvinyl chloride polymer. Since the mechanical integrity of a plastic depends on its high average molecular weight, wear and tear inevitably weakens the material. Weathering degradation of plastics results in their surface embrittlement and microcracking, yielding microparticles that continue on in the environment. Also known as microplastics, these particles act like sponges and soak up Persistent Organic Pollutants (POPs) around them. Thus laden with high levels of POPs, the microparticles are often ingested by organisms in the biosphere.
However, there is evidence that three of the polymers (HDPE, LDPE, and PP) consistently soaked up POPs at concentrations an order of magnitude higher than did the remaining two (PVC and PET). After 12 months of exposure, for example, there was a 34-fold difference in average total POPs amassed on LDPE compared to PET at one location. At another site, average total POPs adhered to HDPE was nearly 30 times that of PVC. The researchers think that differences in the size and shape of the polymer molecules can explain why some accumulate more pollutants than others. The fungus Aspergillus fumigatus effectively degrades plasticized PVC. Phanerochaete chrysosporium was grown on PVC in a mineral salt agar. Phanerochaete chrysosporium, Lentinus tigrinus, Aspergillus niger, and Aspergillus sydowii can effectively degrade PVC.
Plasticizers
Phthalates, which are incorporated into plastics as plasticizers, comprise approximately 70% of the U.S. plasticizer market; phthalates are by design not covalently bound to the polymer matrix, which makes them highly susceptible to leaching. Phthalates are contained in plastics at high percentages. For example, they can contribute up to 40% by weight to intravenous medical bags and up to 80% by weight in medical tubing. Vinyl products are pervasive—including toys, car interiors, shower curtains, and flooring—and initially release chemical gases into the air. Some studies indicate that this outgassing of additives may contribute to health complications, and have resulted in a call for banning the use of DEHP on shower curtains, among other uses. Japanese car companies Toyota, Nissan, and Honda eliminated the use of PVC in car interiors since 2007.
In 2004 a joint Swedish-Danish research team found a statistical association between allergies in children and indoor air levels of DEHP and BBzP (butyl benzyl phthalate), which is used in vinyl flooring. In December 2006, the European Chemicals Bureau of the European Commission released a final draft risk assessment of BBzP which found “no concern” for consumer exposure including exposure to children.
Lead
Lead had previously been frequently added to PVC to improve workability and stability. Lead has been shown to leach into drinking water from PVC pipes.
In Europe (EU 28) the use of lead-based stabilizers was gradually replaced by the end of 2015, under the VinylPlus voluntary commitment, ESPA members completed the replacement of Pb-based stabilizers.
Sustainability
PVC is made from petroleum. The production process also uses sodium chloride. Recycled PVC is broken down into small chips, impurities removed, and the product refined to make pure white PVC. It can be recycled roughly seven times and has a lifespan of around 140 years.
In the UK, approximately 400 tonnes of PVC are recycled every month. Property owners can recycle it through nationwide collection depots. The Olympic Delivery Authority (ODA), for example, after initially rejecting PVC as material for different temporary venues of the London Olympics 2012, has reviewed its decision and developed a policy for its use. This policy highlighted that the functional properties of PVC make it the most appropriate material in certain circumstances while taking into consideration the environmental and social impacts across the whole life cycle, e.g. the rate for recycling or reuse and the percentage of recycled content. Temporary parts, like roofing covers of the Olympic Stadium, the Water Polo Arena, and the Royal Artillery Barracks, would be deconstructed and a part recycled in the Vinyloop process.