Polypropylene
IUPAC Name: Poly (propene)
Chemical formula: (C3H6)n
Melting Point: 130 – 171 Deg C.
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications including packaging and labeling, textiles (e.g., ropes, thermal underwear and carpets), stationery, plastic parts and reusable containers of various types, laboratory equipment, loudspeakers, automotive components, transvaginal mesh and polymer banknotes. An addition polymer made from the monomer propylene, it is rugged and unusually resistant to many chemical solvents, bases and acids.
Polypropylene has a relatively slippery “low energy surface” that means that many common glues will not form adequate joints. Joining of polypropylene is often done using welding processes.
In 2013, the global market for polypropylene was about 55 million tonnes. Polypropylene is the world’s second-most widely produced synthetic plastic, after polyethylene.
Chemical and physical properties
Polypropylene is in many aspects similar to polyethylene, especially in solution behaviour and electrical properties. The additionally present methyl group improves mechanical properties and thermal resistance, while the chemical resistance decreases. The properties of polypropylene depend on the molecular weight and molecular weight distribution, crystallinity, type and proportion of comonomer (if used) and the isotacticity. In isotactic polypropylene, for example, the CH3 groups are oriented on one side of the carbon backbone. This creates a greater degree of crystallinity and results in a stiffer material that is more resistant to creep than both atactic polypropylene and polyethylene.
Mechanical properties
The density of PP is between 0.895 and 0.92 g/cm³. Therefore, PP is the commodity plastic with the lowest density. With lower density, moldings parts with lower weight and more parts of a certain mass of plastic can be produced. Unlike polyethylene, crystalline and amorphous regions differ only slightly in their density. However, the density of polyethylene can significantly change with fillers. The Young’s modulus of PP is between 1300 and 1800 N/mm².
Polypropylene is normally tough and flexible, especially when copolymerized with ethylene. This allows polypropylene to be used as an engineering plastic, competing with materials such as acrylonitrile butadiene styrene (ABS). Polypropylene is reasonably economical. Polypropylene has good resistance to fatigue.
Thermal properties
The melting point of polypropylene occurs at a range, so a melting point is determined by finding the highest temperature of a differential scanning calorimetry chart. Perfectly isotactic PP has a melting point of 171 °C (340 °F). Commercial isotactic PP has a melting point that ranges from 160 to 166 °C (320 to 331 °F), depending on atactic material and crystallinity. Syndiotactic PP with a crystallinity of 30% has a melting point of 130 °C (266 °F). Below 0 °C, PP becomes brittle.The thermal expansion of polypropylene is very large, but somewhat less than that of polyethylene.
Chemical properties
Polypropylene is at room temperature resistant to fats and almost all organic solvents, apart from strong oxidants. Non-oxidizing acids and bases can be stored in containers made of PP. At elevated temperature, PP can be dissolved in nonpolarity solvents such as xylene, tetralin and decalin. Due to the tertiary carbon atom PP is chemically less resistant than PE.
Most commercial polypropylene is isotactic and has an intermediate level of crystallinity between that of low-density polyethylene (LDPE) and highdensity polyethylene (HDPE). Isotactic & atactic polypropylene is soluble in P-xylene at 140 °C. Isotactic precipitates when the solution is cooled to 25 °C and atactic portion remains soluble in P-xylene.
The melt flow rate (MFR) or melt flow index (MFI) is a measure of molecular weight of polypropylene. The measure helps to determine how easily the molten raw material will flow during processing. Polypropylene with higher MFR will fill the plastic mold more easily during the injection or blowmolding production process. As the melt flow increases, however, some physical properties, like impact strength, will decrease.
There are three general types of polypropylene: homopolymer, random copolymer, and block copolymer. The comonomer is typically used with ethylene. Ethylene-propylene rubber or EPDM added to polypropylene homopolymer increases its low temperature impact strength. Randomly polymerized ethylene monomer added to polypropylene homopolymer decreases the polymer crystallinity, lowers the melting point and makes the polymer more transparent.
Degradation
Polypropylene is liable to chain degradation from exposure to heat and UV radiation such as that present in sunlight. Oxidation usually occurs at the tertiary carbon atom present in every repeat unit. A free radical is formed here, and then reacts further with oxygen, followed by chain scission to yield aldehydes and carboxylic acids. In external applications, it shows up as a network of fine cracks and crazes that become deeper and more severe with time of exposure. For external applications, UV-absorbing additives must be used. Carbon black also provides some protection from UV attack.
The polymer can also be oxidized at high temperatures, a common problem during molding operations. Anti-oxidants are normally added to prevent polymer degradation. Microbial communities isolated from soil samples mixed with starch have been shown to be capable of degrading polypropylene. Polypropylene has been reported to degrade while in human body as implantable mesh devices. The degraded material forms a tree bark-like layer at the surface of mesh fibers.
Optical properties
PP can be made translucent when uncolored but is not as readily made transparent as polystyrene, acrylic, or certain other plastics. It is often opaque or colored using pigments.
Industrial processes
Traditionally, three manufacturing processes are the most representative ways to produce polypropylene.
Hydrocarbon slurry or suspension: Uses a liquid inert hydrocarbon diluent in the reactor to facilitate transfer of propylene to the catalyst, the removal of heat from the system, the deactivation/removal of the catalyst as well as dissolving the atactic polymer. The range of grades that could be produced was very limited. (The technology has fallen into disuse).
Bulk slurry (or bulk): Uses liquid propylene instead of liquid inert hydrocarbon diluent. The polymer does not dissolve into a diluent, but rather rides on the liquid propylene. The formed polymer is withdrawn and any unreacted monomer is flashed off.
Gas phase: Uses gaseous propylene in contact with the solid catalyst, resulting in a fluidized-bed medium.
Manufacturing
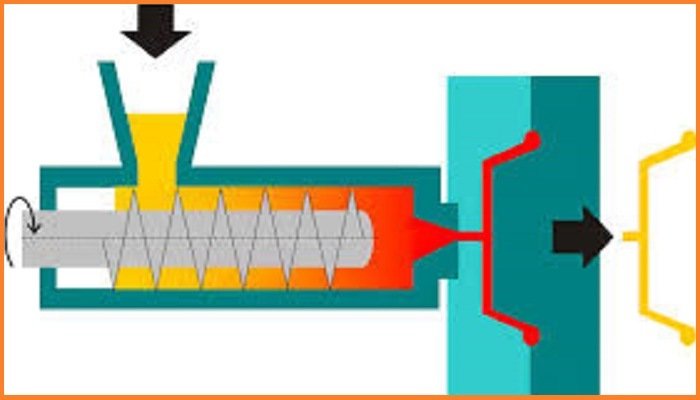
Melting process of polypropylene can be achieved via extrusion and molding. Common extrusion methods include production of melt-blown and spunbond fibers to form long rolls for future conversion into a wide range of useful products, such as face masks, filters, diapers and wipes.
The most common shaping technique is injection molding, which is used for parts such as cups, cutlery, vials, caps, containers, housewares, and automotive parts such as batteries. The related techniques of blow molding and injection-stretch blow molding are also used, which involve both extrusion and molding.
The large number of end-use applications for polypropylene are often possible because of the ability to tailor grades with specific molecular properties and additives during its manufacture. For example, antistatic additives can be added to help polypropylene surfaces resist dust and dirt. Many physical finishing techniques can also be used on polypropylene, such as machining. Surface treatments can be applied to polypropylene parts in order to promote adhesion of printing ink and paints.
Recycling
Polypropylene is recyclable and has the number “5” as its resin identification code.
Application
Household product, EPP model aircraft & structure items etc.