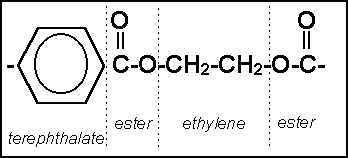
Polyethylene terephthalate
Polyethylene terephthalate is commonly known as PET. PET is the most common thermoplastic polymer resin of the polyester family and is used in fibers for clothing, containers for liquids and foods, thermoforming for manufacturing, and in combination with glass fiber for engineering resins. Chemical formula is (C10H8O4)n.
PET is 04th largest used thermoplastic polymer, which mostly used in making bottles. As on ranking PE is the First, PP is the Second and PVC is the third most used thermoplastic polymer in world.

Physical Properties:
PET in its natural state is a colorless, semi-crystalline resin. Based on how it is processed, PET can be semi-rigid to rigid, and it is very lightweight. It makes a good gas and fair moisture barrier, as well as a good barrier to alcohol (requires additional “barrier” treatment) and solvents. It is strong and impact-resistant. PET becomes white when exposed to chloroform and also certain other chemicals such as toluene.
| Properties | Nominal Values |
| Density | 1.38 – 1.45 gm/cc |
| Tensile strength | 55 – 70 MPa |
| Elastic Modulus | 2800 – 3100 MPa |
| Elongation | Min. 50% |
| Izod Notch test | 3.6 Kj/m2 |
| Vicat Softening Temp. | Min. 82 Deg C |
| Water Absorption | Max. 0.16 |
| Glass Transition Temp. | 70 – 80 Deg C |
| Melting Temperature | 240 – 260 Deg C |
| Boiling Point | > 350 Deg C |
Viscosity Range of PET:
Fiber grade:
0.40–0.70 Textile
0.72–0.98 Technical, tire cord
Film grade:
0.60–0.70 BoPET (biaxially oriented PET film)
0.70–1.00 Sheet grade for thermoforming
Bottle grade:
0.70–0.78 Water bottles (flat)
0.78–0.85 Carbonated soft drink grade
Monofilament, engineering plastic
1.00–2.00
Production
Polyethylene terephthalate is produced from ethylene glycol and dimethyl terephthalate(DMT) (C6H4(CO2CH3)2) or terephthalic acid.
The former is a transesterification reaction, whereas the latter is an esterification reaction.
Dimethyl terephthalate process (DMT)
In dimethyl terephthalate(DMT) process, this compound and excess ethylene glycol are reacted in the melt at 150–200 °C with a basic catalyst. Methanol (CH3OH) is removed by distillation to drive the reaction forward. Excess ethylene glycol is distilled off at higher temperature with the aid of vacuum. The second transesterification step proceeds at 270–280 °C, with continuous distillation of ethylene glycol as well.
Degradation
PET is degraded at various type during processing. The main degradations that can occur are hydrolytic, and probably most important, thermal oxidation. When PET degrades, It leads: discoloration, chain scissions resulting in reduced molecular weight, formation of acetaldehyde, and cross-links (“gel” or “fish-eye” formation). Discoloration is due to the formation of various chromophoric systems following prolonged thermal treatment at
elevated temperatures. This becomes a problem when the optical requirements of the polymer are very high, such as in packaging applications. The thermal and thermos-oxidative degradation results in poor processibility characteristics and performance of the material.
Recycling:
PET is fully recycling thermoplastic material.
Application & Uses:
- PET is an excellent water and moisture barrier material, plastic bottles made from PET are widely used for soft drinks. For certain specialty bottles, such as those designated for beer containment & daily using bottles.
- Biaxially oriented PET film (often known by one of its trade names, “Mylar”) can be aluminized by evaporating a thin film of metal on to it to reduce its permeability, and to make it reflective and opaque. Also used to make high strength Tapes, Flexible food packaging & Thermal insulation etc..
- Non-oriented PET sheet can be thermoformed to make packaging trays and blister packs.

(flexible food packaging box)



good information.
Thanks a lot. For your appreciation.
thanks for appreciation
is it possible increasing Tg of PET by compounding with out reduction transparency
First of all thank you for visiting this site. of course we can increase with using high quality impact modifier.
Thanks for the wonderful guide
thank you so much
Great article.i have two questions.1,what thermoolastic is compatiable to pet if needs to mix in a small %..2.i want to learn the process of making pet base colour masterbatch.need advice.
hi.
thanks about your Description
i want to know how we can product Soda Glass from pet.
thank you